Data Standards Governor
Your centralized metadata repository and data standards governance platform
Backstory: Connected Standards Enablement Initiative
A clinical data management group inside a large pharma company approached us to help them improve the way they manage the necessary connections between their clinical standards. This group manages standards on different levels and ranging from data collection (CRF, Data Transfer Agreements) to data delivery (SDTM) and analysis (ADaM).
The standards were maintained as file-based tables. ("Metadata management by Excel files," as they put it).
Determining the impact of changes in connected standards is tedious, time-consuming, and often manual, making it expensive and prone to human error.
In the pharma industry compliance is a submission requirement:
- submission data sets are expected to consistently apply CDISC terminology where available
- sponsors are expected to define and consistently apply additional terminologies when not available in CDISC terminology
We developed a turnkey system that enables our client to store and manage the connected standards.
The solution is already delivering benefits for the standards team, while also enabling the output of data in a format that integrates with the client's current downstream processes.
The system delivers data to a larger audience in a user-friendly way, while also automating its ingestion by downstream tools like conversion and data review applications.
Our Solution
This is a high-level view of the benefits our system delivers to our client.
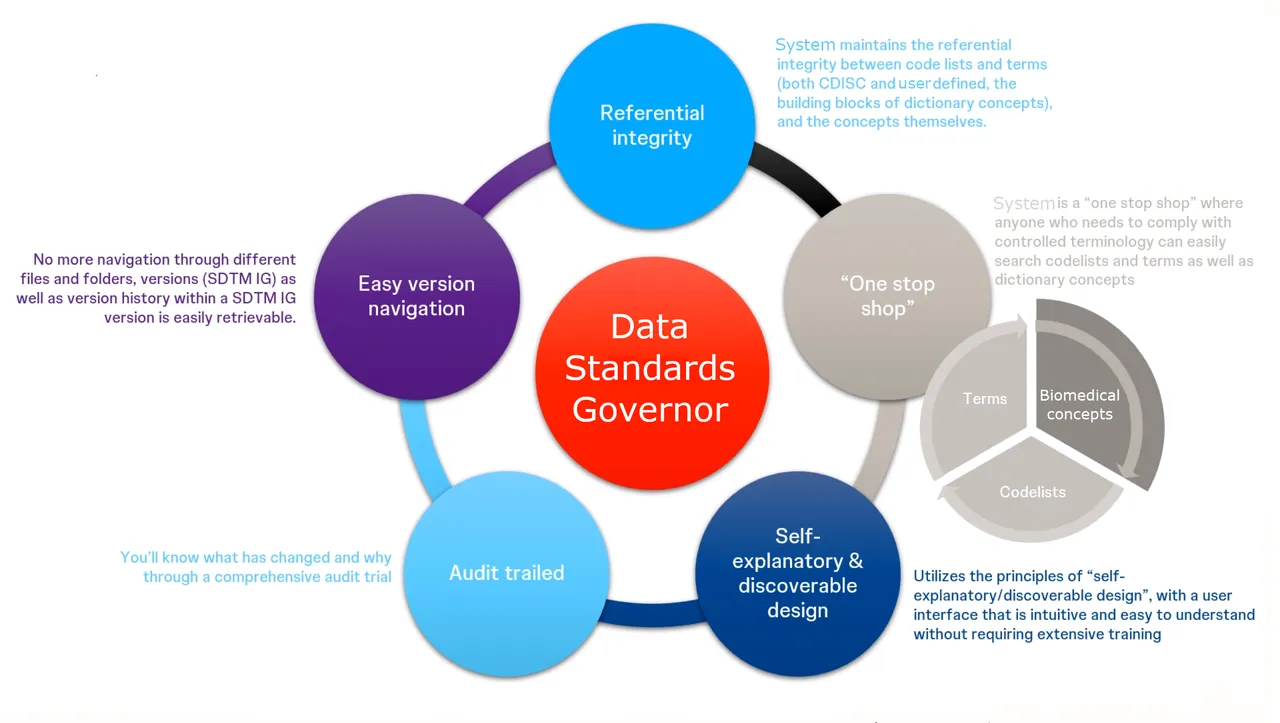
Summary:
- ✓ Near real-time content management and availability
- ✓ Single source for connected, reusable and easy-to-find clinical terminology and concepts
- ✓ Easier and faster to be compliant with regulatory requirements
The system is API-first, with a robust role-based access control mechanism. And we're fully integrated into standards SSO platforms (i.e. Microsoft's Entra and Okta).
Our system is a SaaS platform that stores, curates, and governs industry-standard terminologies, code-sets, and reference data needed for regulatory submissions (drugs, devices, and diagnostics).
Client avoids the "terminology drift" problem when different synonyms, and/or outdated codes are used in studies.
Technical Details
Now, these are specific details on the system's capabilities.
Integrated Backups
System has built-in backup capabilities to safely make backup copies of the system's state at any point in time, roll back the system's content to a previous state, and authorized users can perform both backup and restore actions.
- ✓ Safely backup system state
- ✓ Roll back to previous state
- ✓ Authorized user access
- ✓ Point-in-time recovery
Content Catalog
The system can handle a wide variety of what we call "Item Types." Each Item Type is defined internally using JSON, giving system an almost limitless ability to handle from the simple ones to the most complex data types: Terms, Biomedical Concepts, CRFs, Libraries, and Studies.
- ✓ JSON-based item definitions
- ✓ Flexible data types
- ✓ Term management
- ✓ Biomedical concepts
Dashboards
System provides multiple visual displays of information. Each dashboard can be easily customized to better fit a client's needs.
- ✓ Customizable dashboards
- ✓ Real-time data visualization
- ✓ Multiple display options
- ✓ Client-specific views
Document Repository
System is able to handle documents (Excel, PDFs, Word) with full version control. And each document can be tied to one or many terms or concepts in any of the available collections.
- ✓ Full version control
- ✓ Multiple document types
- ✓ Link to terms/concepts
- ✓ Document management
Impact Assessment
One of the defining features of the system is the ability of a user to visually see the impact of a proposed change. When editing a codelist, the system graphically displays all related items impacted by the intended change (addition, modification, or deletion).
- ✓ Visual impact analysis
- ✓ Related items display
- ✓ Change preview
- ✓ Dependency tracking
Item-level History & Releases
Authorized users can "package" versions of content into date-based releases to better match business cycles.
- ✓ Date-based releases
- ✓ Version packaging
- ✓ Business cycle alignment
- ✓ Release management
Item-level Audit Trail
The system is designed to capture Who did What When How at the item level.
- ✓ Complete audit trail
- ✓ User action tracking
- ✓ Timestamp recording
- ✓ Change documentation
Item-level Updates
Each item can be updated, and system will guide user to fill out the required fields (as defined by the item-level metadata).
- ✓ Guided updates
- ✓ Required field validation
- ✓ Metadata-driven forms
- ✓ User-friendly interface
Item-level Dependencies
Depending on its metadata definition, an item can have dependencies on other items.
- ✓ Dependency management
- ✓ Metadata-driven links
- ✓ Impact tracking
- ✓ Relationship validation
Item-level Relationships
Depending on its metadata definition, an item can have relationships with other items.
- ✓ Relationship management
- ✓ Connected items
- ✓ Metadata-based links
- ✓ Cross-references
Metrics
System provides authorized users with detailed metrics in terms of usage patterns, tasks, content volume, etc.
- ✓ Usage patterns
- ✓ Task metrics
- ✓ Content volume tracking
- ✓ Performance analytics
Reports
System is pre-loaded with default reports. Additional reports can be easily created to fit a client's needs.
- ✓ Default report templates
- ✓ Custom report creation
- ✓ Client-specific reports
- ✓ Export capabilities
SDTM Model
This is a screenshot of SDTM data stored in system.
- ✓ CDISC SDTM compliance
- ✓ Standard domains
- ✓ Variable definitions
- ✓ Controlled terminology
Task Management
System has a built-in task management system to assist content editors in the management and approval of content changes.
- ✓ Task assignment
- ✓ Approval workflows
- ✓ Content change tracking
- ✓ Editor collaboration
Business Benefits
Clients that use our system benefit through:
- ✓ Significant time savings (no more scouting websites and fetching Excel files)
- ✓ Peace of mind that their organization uses the correct data and terminology standards
- ✓ A single, unified platform for all relevant standards: industry-defined as well as client-specific
- ✓ Automated terminology alignment between industry and client-defined terms
- ✓ The ability to move to different versions of standards (ie. SDTM) at the client's pace
- ✓ Allowing individual trials to start and use different versions of standards and terminologies
- ✓ A clear view of standard changes to determine what to do to upgrade trial data to new standard
- ✓ A comprehensive Data Governance process: version control, traceability, and full provenance
- ✓ The ability to augment industry standards with client-specific standards and terminologies
- ✓ Use of platform for post-clearance submissions as well (i.e. standards-compliant ICSRs)
- ✓ A fully hands-off, done-for-you service available immediately
We presented our system's vision at the PHUSE EU Connect 2023 event with our Metadata-Driven Platform paper.
Download PHUSE Paper (PDF)Contact Us
Please contact us for more details about Data Standards Governor.